| Charles Karemangingo Ph. D. P. Ag. | DAFA-2004-P001 |
WHAT IS SOIL PHOSPHORUS?
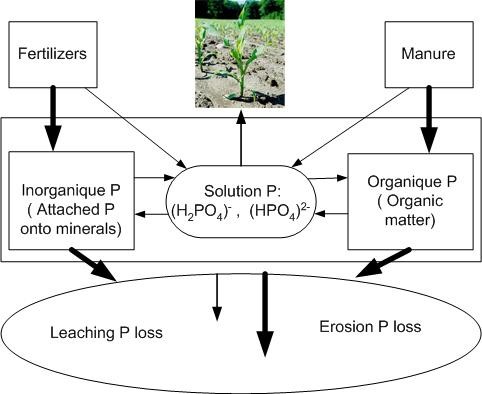
Phosphorus (P), like nitrogen and potassium, is an essential nutrient for plant growth. It is a critical plant nutrient because of its low concentration in soils ( 600 ppm total P) and its low solubility (average of 0.05 mg P L-1 in soil solution). Soil P exists in inorganic and organic forms (Fig. 1). Inorganic forms are associated with amorphous and crystalline aluminium and iron compounds in acid soils and calcium compounds in alkaline soils. Organic P forms are associated with organic materials in soil.
Continuous P exchanges take place between various P forms. Plants absorb phosphorus from the soil solution. Soil solution P concentration decreases with the increase of plant P uptake, P fixation on soil minerals, and micro-organism activities. That decrease triggers P desorption from mineral P forms. In addition, the mineralization of plant residue P under microbial activity increases available soil phosphorus (both labile and dissolved forms). All soil P forms also change when P fertilizers or manures are applied. All the P forms may also be lost through soil erosion and leaching processes.
WHAT DOES PHOSPHORUS DO?
The most essential role of phosphorus in plants is to store energy and provide it again plant cells when they needed it. This is done through complex organic molecules commonly referred to as ADP (adenosine diphosphate) and ATP (adenosine triphosphate). These two compounds are formed and regenerated in the presence of sufficient phosphorus. Phosphorus is also an important structural component of nucleic acids (DNA or deoxyribonucleic Acid, and RNA or Ribonucleic acid), coenzymes, phosphoproteins, phospholipids, and sugar phosphates.